Michael
Pharmacist Driven Shingrix Follow-Up, a Randomized Controlled Trial
April 22, 2020 in College of Pharmacy, Virtual Poster Session Spring 2020
Background
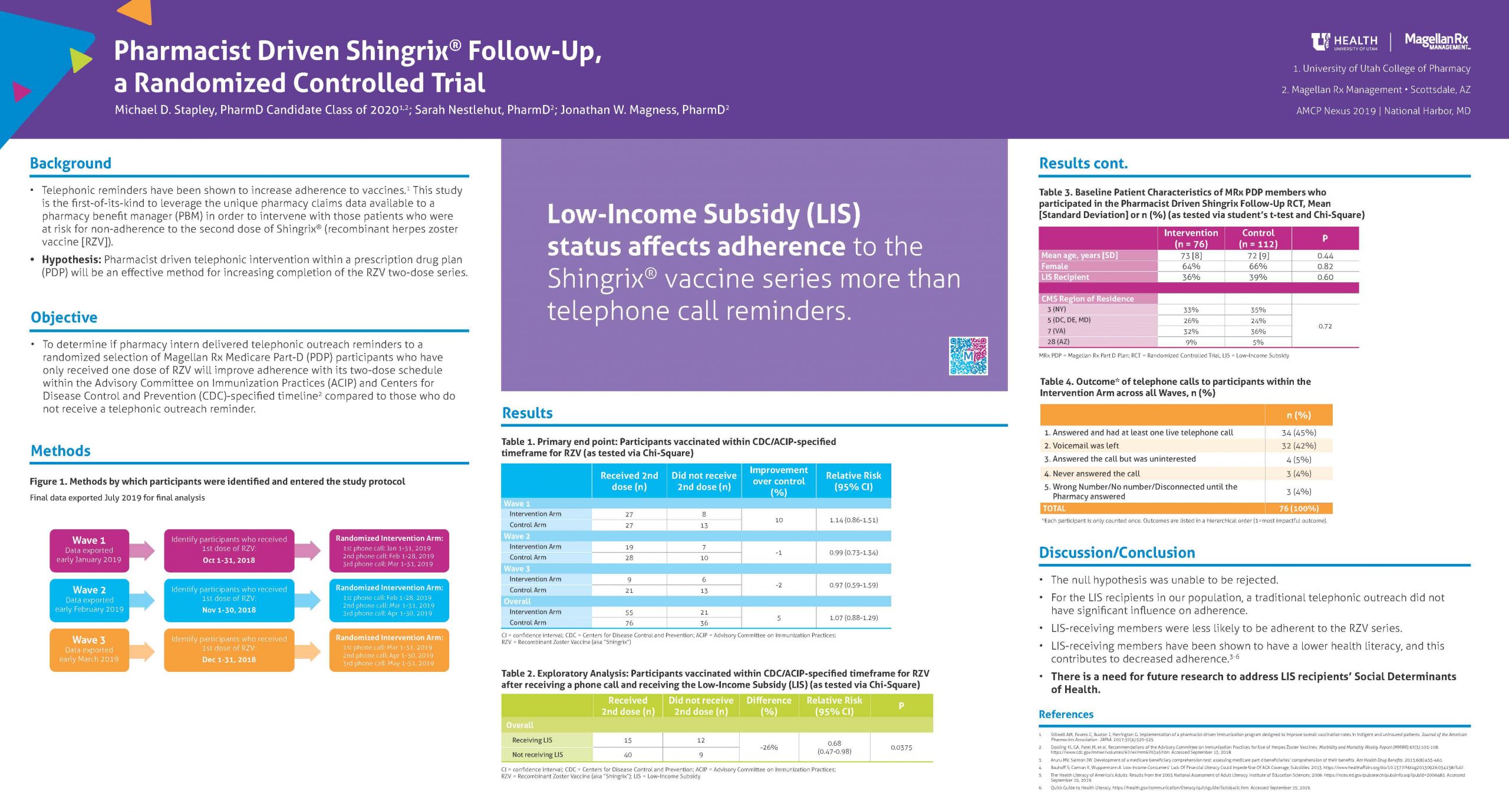
Herpes zoster is a vaccine-preventable disease. The CDC and ACIP recently updated guidelines to include Recombinant Zoster Vaccine (RZV or Shingrix) as the preferred vaccine over Zostavax or Zoster Vaccine Live (ZVL). Despite RZV’s increased efficacy, one of its limitations is that it requires two doses compared to ZVL’s single dose. Pharmacy Benefit Managers (PBMs) can leverage the claims data available to them to perform pharmacist/pharmacy intern driven telephonic follow-up to increase adherence to the series and prevent both costly and painful herpes zoster and its secondary effects.
Objective
To determine if pharmacist/pharmacy intern delivered telephonic outreach reminders to a randomized selection of Magellan Rx Medicare Prescription Drug Plan (PDP) participants who had only received one dose of RZV would improve participant adherence with its two-dose vaccine schedule compared to those who did not receive a telephonic outreach reminder.
Methods
A total of 188 Magellan Rx PDP (MRx PDP) beneficiaries were blinded and randomized into the intervention (n = 76) and control (n = 112) arms. Each beneficiary had only received one dose of RZV at randomization. The study consisted of three “waves,” and phone calls were made to all intervention arm participants when they were 3, 4, and 5 months removed from their initial dose. Final pharmacy claims data were exported in early July 2019, and all participants were reviewed per-protocol to identify whether or not they completed the RZV series.
Results
Compared to control, the intervention group saw a non-significant increase of 4.5% (p = 0.51) in the overall proportion adherent to the complete RZV series. Nine participants in total received their second dose outside of the recommended 6-month time window. In exploratory analyses, receiving a low-income subsidy (LIS) negatively affected adherence to the second dose in both arms of the study, with a composite relative risk of 0.70 (95% CI: 0.55-0.88).
Conclusions
This study lacked sufficient sample size and power for the primary outcome result to be statistically significant. However, in post-hoc analyses, members were significantly less likely to be adherent to the RZV series if they also received a LIS compared to those that did not receive the subsidy. LIS-receiving members have been shown to have a lower health literacy, and this may have contributed to their decreased adherence. There is need for future research to address LIS recipients’ social determinants of health.
Sponsorship
This work was sponsored by Magellan Rx Management, then employer for Stapley, Nestlehut, and Magness.
