Gillins David
Testing Novel Steroid-Targeting Therapies for Tourette Syndrome in Transgenic Mouse Models
April 28, 2020 in College of Pharmacy, Virtual Poster Session Spring 2020
Abstract
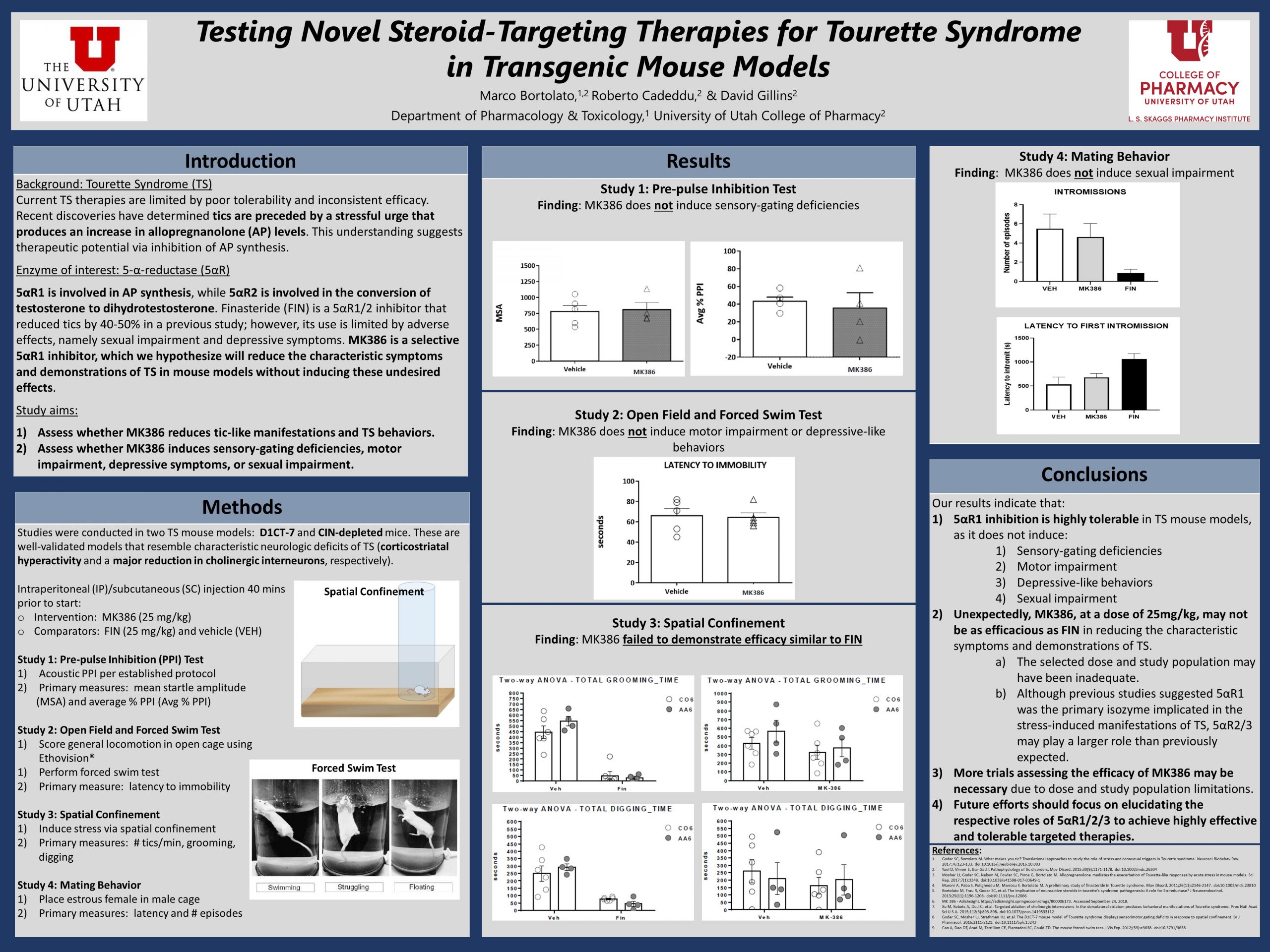
Tic management in Tourette syndrome (TS) is a significant challenge as available pharmacotherapies have limited efficacy and troubling adverse effects. Recent discoveries indicate that stress-induced production of the neurosteroid allopregnanolone (AP) plays a critical role in facilitating tic emergence. Finasteride (FIN), a non-selective AP synthesis inhibitor, has been demonstrated to substantially decrease tic frequency; however, it’s lack of selectivity often leads to adverse effects. This suggests therapeutic potential for a highly selective inhibitor of the main isozyme that produces AP, such as MK386. Our objective was to determine whether 1) MK386 reduces tics in TS mouse models without 2) inducing significant adverse effects. Studies were conducted in adult male wild-type mice (C57BL6/J) and transgenic mouse models (D1CT-7 and CIN-depleted mice). Mice were divided among treatment groups and administered MK386, FIN, or a solvent vehicle. A series of tests were performed to assess tolerability and effectiveness, including acoustic pre-pulse inhibition, open field observation, forced swim, and spatial confinement. Data was collected by a trained observer and analyses were conducted to determine the statistical difference between treatment groups. We found that treatment with MK386 does not induce sensory-gating deficiencies, motor impairment, depressive-like behaviors, or sexual impairment; however, our findings also indicate that MK386, at a dose of 25 mg/kg, fails to significantly reduce tics in TS.
