Jennifer
Characterization of Biorecognizable Elastinlike Polypeptides
April 28, 2020 in College of Pharmacy, Virtual Poster Session Spring 2020
Abstract
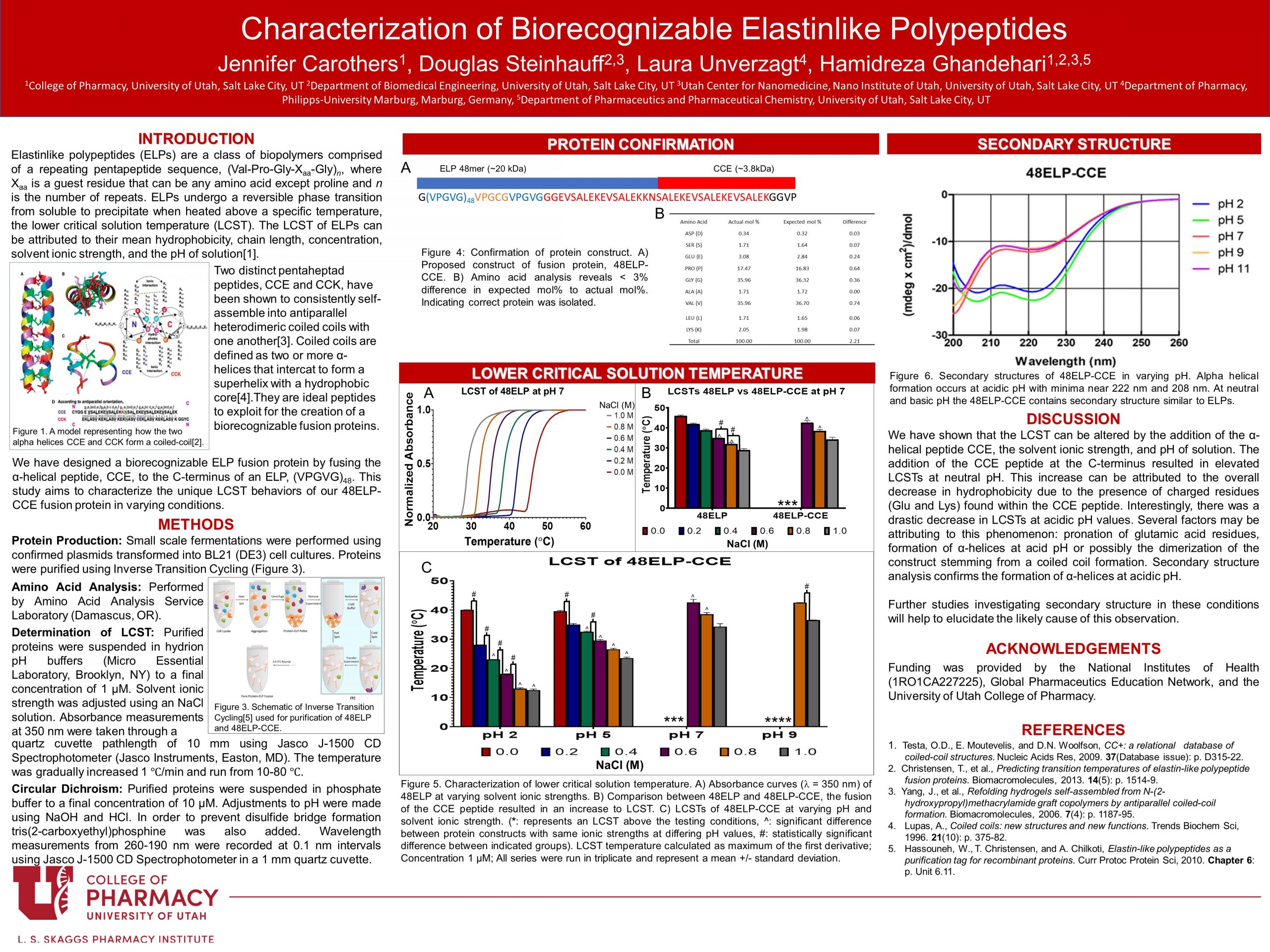
Despite advances in targeted immunotherapies, many patients suffering from non-Hodgkin’s lymphoma continue to be resistant to standards of treatment. Novel two-step nanotherapies, drug free macromolecular therapeutics (DFMTs), are currently being investigated as an alternative. Current DFMTs utilize synthetically produced polymers fused to a pair of α-helical peptides, CCE and CCK. Synthetic polymers may lead to an unpredictable response of the overall system. We have designed a similar system utilizing a thermoresponsive biopolymer, elastinlike polypeptide (ELP), fused to the α-helical peptide, CCE. ELPs have a unique reversible physical property of phase transitioning from soluble to precipitate when heated above a specific temperature, the lower critical solution temperature (LCST). The goal of this study is to expand our understanding of how the fusion of a single CCE domain to a 48-mer ELP affects LCST properties and secondary protein structures in varying conditions. LCST and secondary structure were investigated using standard turbidimetry and circular dichroism spectroscopy processes. The fusion of CCE to 48-mer ELP resulted in an increase in LCST. Increases to LCST were also observed at lower solvent ionic strengths and more basic pH values. Secondary structure analysis showed α-helical formation in acidic pH, correlated to drastic decrease in LCST. We have shown that the LCST can be altered by the addition of the α-helical peptide CCE, solvent ionic strength, and the solvent pH. This data will serve as a foundation for rational development of an ELP based drug free macromolecular therapeutic.
