Hansen Taylor
Evaluating synergy of belinostat and ribociclib in triple-negative breast cancer
April 27, 2020 in College of Pharmacy, Virtual Poster Session Spring 2020
Abstract
Purpose:
Triple Negative Breast Cancer (TNBC) has a poor prognosis due to higher rates of metastases and a lack of targeted treatments. Emerging targeted therapies currently being studied include Histone Deacetylase Inhibitors (HDACi) and Cyclin Dependent Kinase Inhibitors (CDKi). We hypothesize that a synergistic effect will occur in the treatment of TNBC when using Belinostat (an HDACi) and Ribociclib (a CDKi). We propose that the mechanism of synergy between Belinostat and Ribociclib is through the Retinoblastoma protein (Rb) pathway. Evidence suggests that HDACi increases levels of endogenous CDKi thereby increasing the level of active Rb which arrests cell cycle progression from the G1 to the S phase.
Methods:
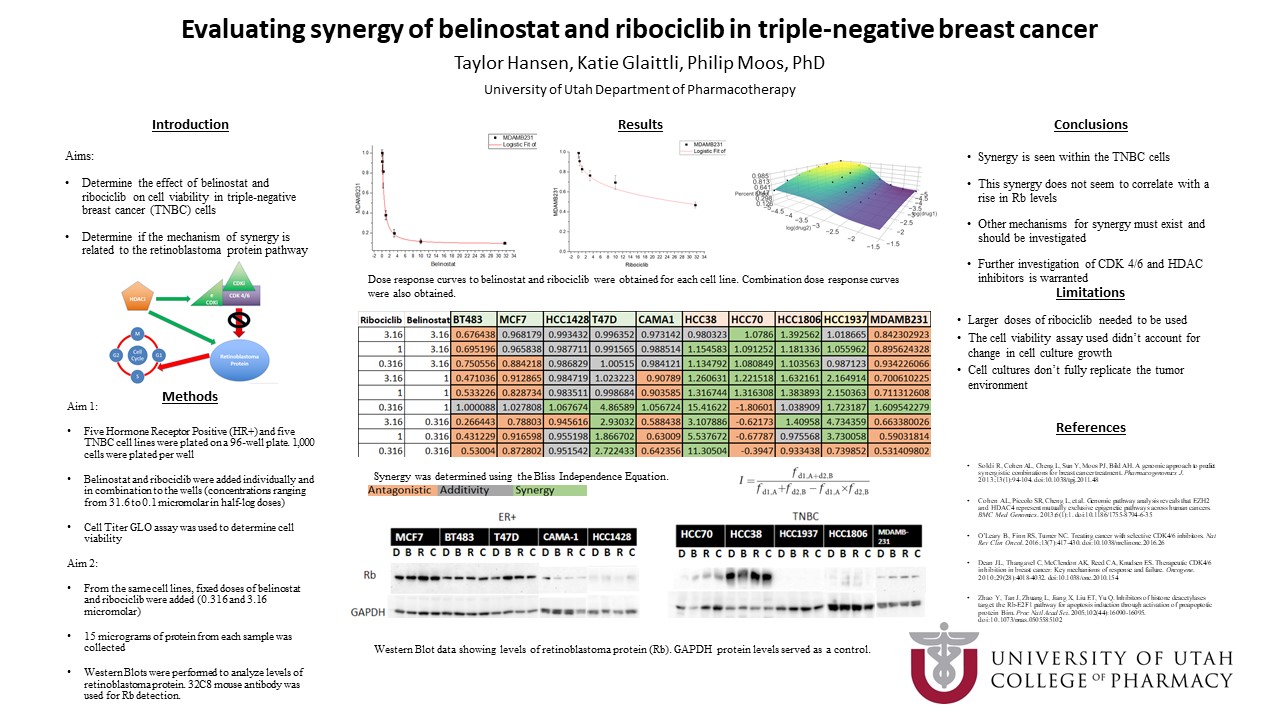
In this study, we used five hormone receptor-positive (HR+) breast cancer cell lines and five TNBC cell lines. 1,000 cells of each line were plated per well and grown in 3-D culture in a 96 well plate. Cells incubated for 24 hours until spheroids formed. Six doses of Belinostat and Ribociclib from 0.316 to 31.6 micromolar and nine combination doses were added to the cells. After 72 hours of drug incubation, CellTiter GLO was used to determine cell viability by measuring ATP. Dose-response curves for the individual drugs were obtained and the Bliss Independence Equation was used to determine if synergy was achieved with the drug combinations. Synergy within a cell line was determined if at least 4 of the 9 combination doses in each line showed synergy through the Bliss Independence Equation.
In order to delve into the proposed mechanism, we looked at Rb levels of each cell line. 8,000 cells of each line were plated on their own 96 well plate. After spheroids formed, drugs were treated with the 50% Effective Concentration (EC50) dose of Belinostat and Ribociclib (both separately and in combination) or DMSO for 24 hours. Cells were then lysed and protein from each sample was collected. 15 nanograms of protein from each sample were run by gel electrophoresis. Western Blots were performed to view levels of Rb.
Results:
Dose-response curves were obtained for all ten cell lines. We found that synergy was achieved in one out of five HR+ cell lines (T47D cell line) and four out of five TNBC cell lines (HCC38, HCC70, HCC1806, and HCC1937 cell lines).
Based on the obtained EC50, the combination doses used to determine levels of Rb were 0.316 micromolar of Belinostat and 3.16 micromolar of Ribociclib. Rb levels were elevated with drug treatment in three of the five TNBC cell lines (HCC38, HCC1806, and MDA-MB 231) indeterminate in one cell line (HCC70) and nonexistent in the last (HCC1937). Genetic data shows that the HCC1937 cell line has a genetic mutation in the Rb gene thereby explaining the lack of the protein in our Western Blots. Rb levels were elevated with drug treatment in four of the five ER+ cell lines (MCF7, BT483, T47D, and HCC1428) and indeterminate in one cell line (CAMA-1).
Conclusion:
Cell lines that showed synergy and an increase in Rb were the T47D (ER+), HCC38 (TNBC), and HCC1806 (TNBC) cell lines. Even with a lack of Rb, synergy was still seen in the HCC1937 cell line (TNBC).
From our results, we can see synergy in TNBC cell lines. However, this synergy does not seem to correlate solely with a rise in Rb protein levels. Other mechanisms for synergy between Belinostat and Ribociclib may exist and need further exploration to find other potential biomarkers. From these experiments, we conclude that synergy is commonly seen in TNBC cells and that further clinical trials with Belinostat and Ribociblib warrant investigation.
