Synthesis and PTP inhibitory activity of illudalic acid and its methyl ether, with insights into selectivity for LAR PTP over other tyrosine phosphatases under physiologically relevant conditions
Abstract
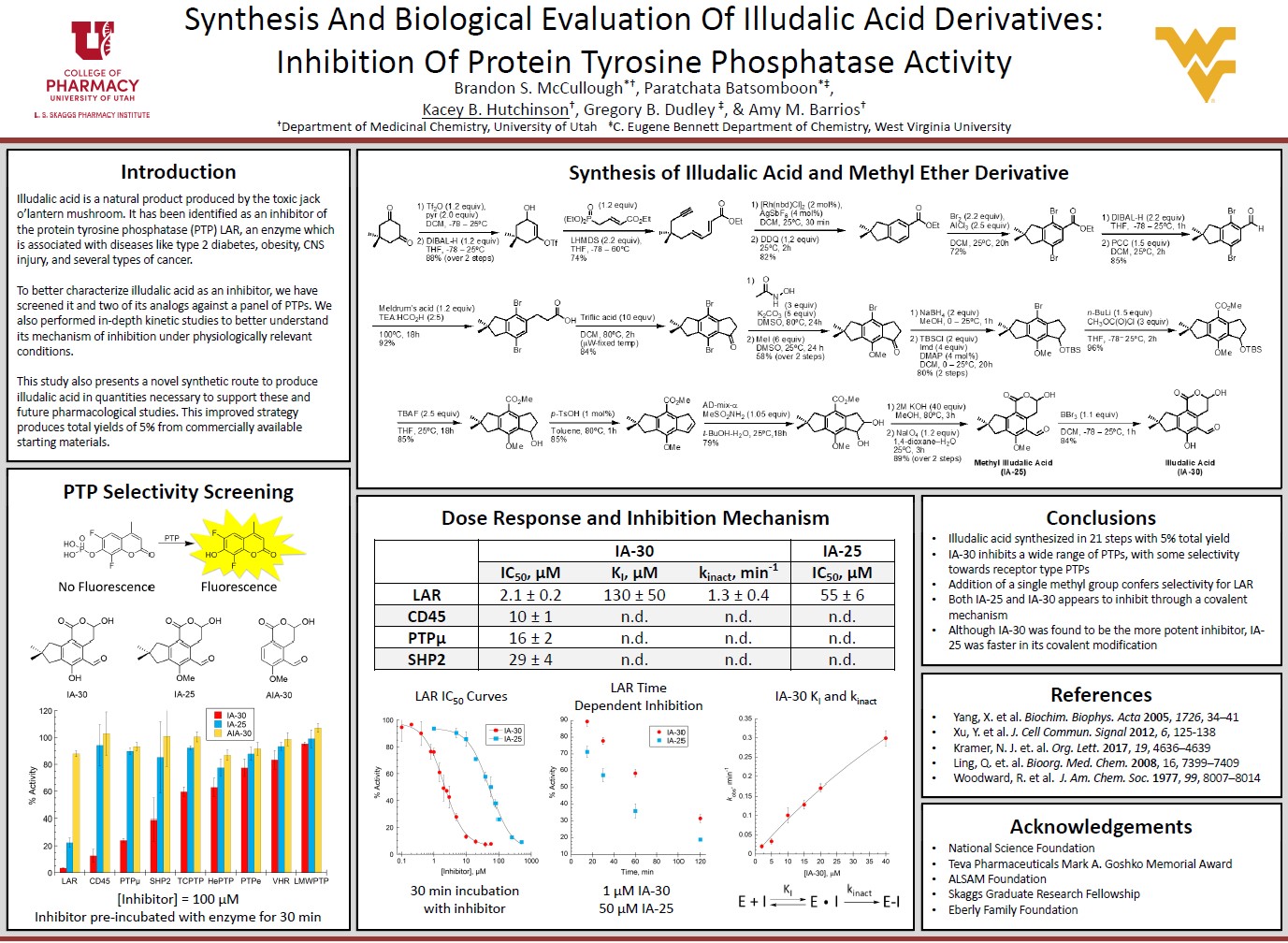
The protein tyrosine phosphatase (PTP) family of enzymes includes many attractive therapeutic targets, such as those in the leukocyte common antigen-related (LAR) subfamily of receptor PTPs. Synthesis and PTP inhibitory activity of illudalic acid and its methyl ether are described, with a focus on selective inhibition of LAR PTP relative to a small collection of other representative PTPs. The synthesis comprises 16 steps and provides illudalic acid in up to 12% overall yield from neopentylene-fused benzoate 1 (20 steps from commercial materials). Illudalic acid dose-dependently (measured IC50 = 2.1 ± 0.2 uM) and time-dependently inhibits LAR consistent with previous reports of covalent binding. The kinetics of LAR inhibition by illudalic acid are consistent with a two-step mechanism in which the inhibitor and enzyme first interact non-covalently (KI = 130 ± 50 µM), followed by covalent ligation at a rate kinact = 1.3 ± 0.4 min-1. The kinact/KI ratio of 104 corresponds to a t∞1/2 of 0.5 min, as discussed herein. The phenol methyl ether of illudalic acid was found to be less potent in our dose-response assays (measured IC50 = 55 ± 6 uM) but more selective for LAR, with a weaker initial non-covalent interaction and faster covalent ligation of LAR as compared to illudalic acid itself. A truncated analog of illudalic acid that lacks the neopentylene ring fusion was found to be devoid of significant activity under our assay conditions, in contrast to previous reports. These observations collectively help inform further development of illudalic acid analogs as potent and selective inhibitors of the LAR subfamily of tyrosine phosphatases.
Published in College of Pharmacy, Virtual Poster Session Spring 2020
Since I know you finished up your project pretty early, do you know what the lab has planned as a follow up, if anything? I know the plans may be on hold due to COVID19, but it would be interesting to see where this goes next.
The project sets the stage for more research involving illudalic acid and its analogs as potential LAR inhibitors. Future studies could potentially study analogs not used in this study or use the knowledge gained to create more specific small molecule LAR inhibitors. Steps much further down the line could potentially include animal studies to see if LAR inhibition influences related disease states in vivo. I know the Barrios lab is still working with LAR (and other PTPs) and small molecule inhibitors. I unfortunately have not been involved in additional research in the lab so I’m not sure exactly how much progress has been made. I’ll look into it for you!
Kacey, very nice work. What part of the project, specifically, were you involved in? I am excited about this, as we have a grant under review to focus on PTPRdelta in the context of addiction. It is my hope that in collaboration with Dr. Barrios and others we can find PTPRdelta inhibitors that may be useful in preventing the development of drug addiction!
Thank you! I was involved in everything except the synthesis portion. We partnered with another lab group who focused on creating a more efficient synthesis pathway for illudalic acid and its analogs. They supplied us with those compounds so we could use them as small molecule inhibitors in my project. I was directly involved in obtaining the data needed for the project. I spent a lot of time in the lab doing initial inhibition screens, running assays to make IC50 curves, and just getting the data needed to write the article. I am very grateful that we got some interesting results!
I was actually planning on working with PTPRdelta for my PharmD project! Unfortunately, we weren’t able to get that specific phosphatase in time. Good luck with the grant and the project! I almost wish pharmacy school was a little longer so I could be a part of that too! I think the topic is very interesting!