Tigh Jeremy
Generating Antibodies Against P. aeruginosa
April 29, 2020 in College of Pharmacy, Virtual Poster Session Spring 2020
Abstract
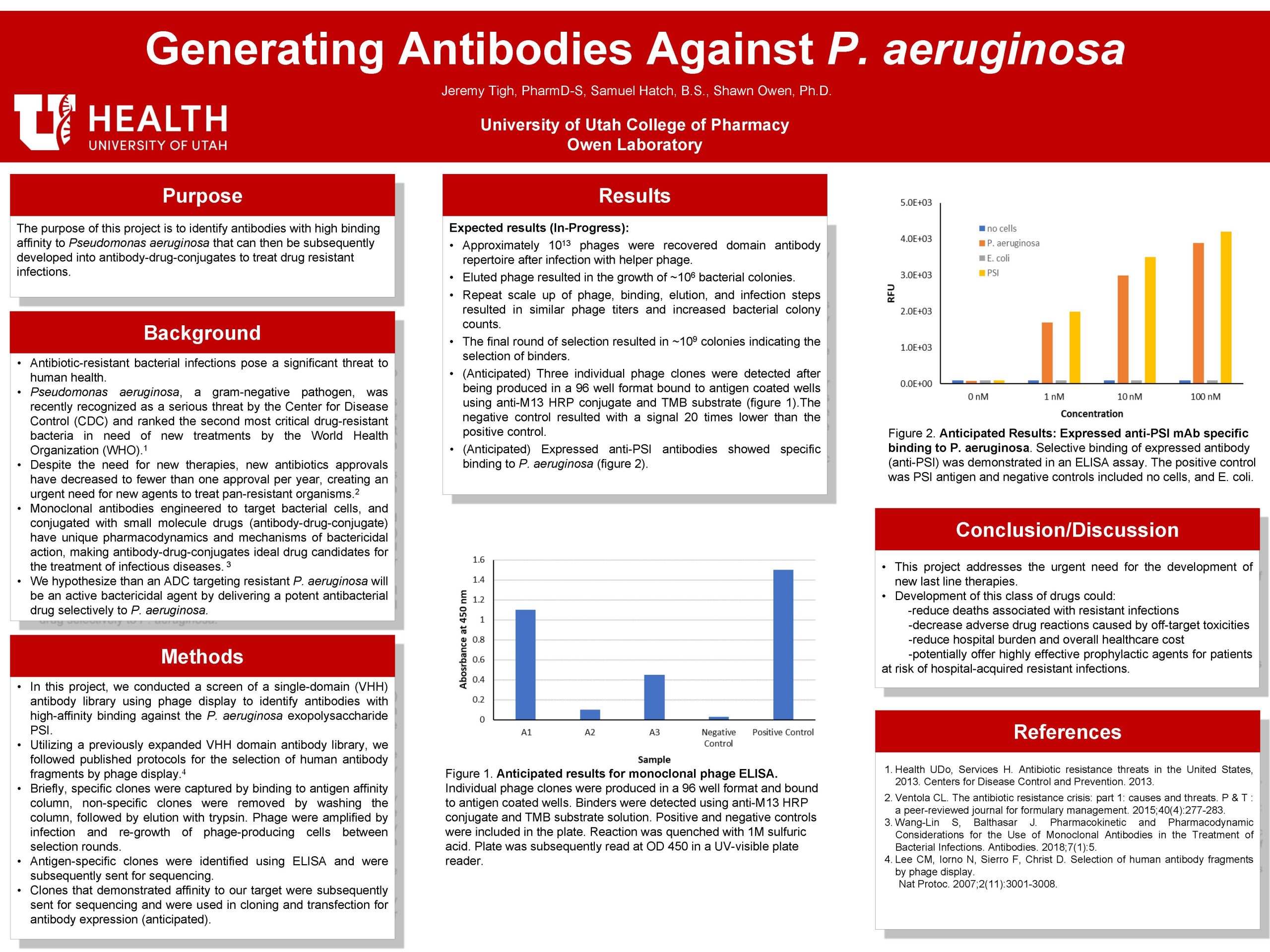
Introduction: Antibiotic resistant bacterial infections pose a significant threat to human health. Pseudomonas aeruginosa, a gram-negative pathogen, was recently recognized as a serious threat by the Center for Disease Control (CDC), and ranked the second most critical drug resistant bacteria in need of new treatments by the World Health Organization (WHO). Despite the need for new treatments, new antibiotics approvals have decreased to fewer than one approval per year, creating an urgent need for new agents to treat pan resistant organisms. Monoclonal antibodies engineered to target bacterial cells, and conjugated with small molecule drugs (antibody-drug-conjugate) have unique pharmacodynamics and mechanisms of bactericidal action, making antibody-drug-conjugates ideal drug candidates for the treatment of infectious diseases. We hypothesize than an ADC targeting resistant P. aeruginosa will be an effective bactericidal agent by delivering a potent antibacterial drug selectively to P. aeruginosa. The purpose of this project is to identify antibodies with high binding affinity to P. aeruginosa that can then be subsequently developed into antibody-drug-conjugates to treat resistant P. aeruginosa.
Materials and Methods: We conducted a high-throughput screen of a single-domain (VHH) antibody library using antibody phage display following published protocols to identify antibodies with high affinity binding against the P. aeruginosa exopolysaccharide Psl. Recovered clones were subsequently sequenced. Recovered sequences were then cloned into expression vectors and antibodies were expressed in CHO cell culture. Binding of the resulting anti-Psl mAbs was verified by ELISA assay and in cell culture against P. aeruginosa.
Results and Discussion: Expected results- Using antibody phage display we identified three clones that demonstrated high affinity binding to our antigen of interest. Sequences for each clone were recovered and submitted to the public domain. Anticipated: Following cloning and transfection, expression of the subsequent antibody demonstrated selective binding to P. aeruginosa. This project demonstrates the ability to use an antibody phage library to screen for and select sequences that bind to a known antigen.
Conclusions: This project addresses the urgent need for the development of new last line therapies. Development of this class of drugs could reduce deaths associated with resistant infections, decrease adverse drug reactions caused by off target toxicities, reduce hospital burden and overall healthcare cost, and potentially offer highly effective prophylactic agents for patients at risk of hospital acquired resistant infections.
