Lydia
Characterization of adverse effects of GLP-1 receptor agonist exposures reported to US Poison Centers
April 29, 2020 in College of Pharmacy, Virtual Poster Session Spring 2020
Abstract:
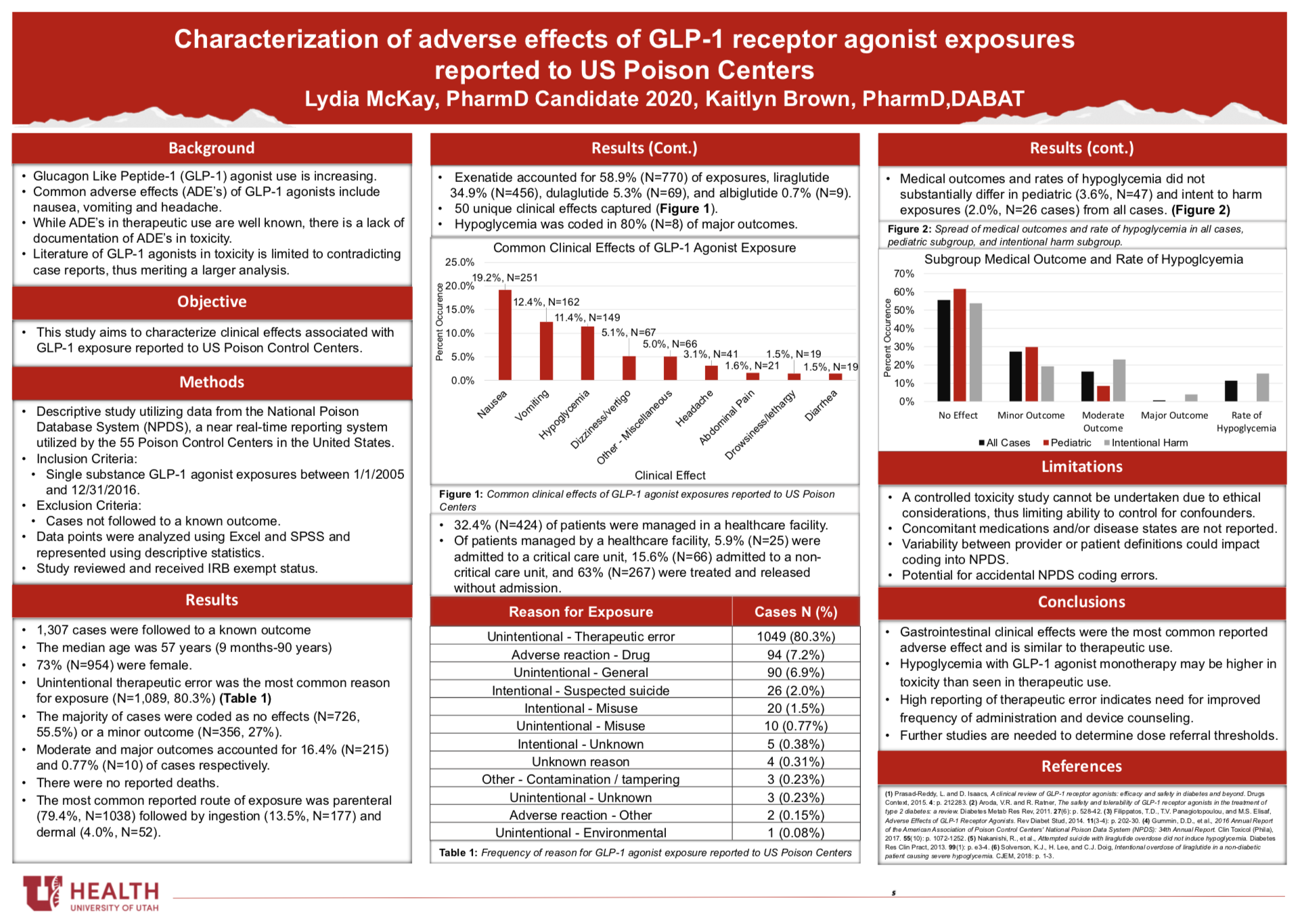
Background: Glucagon Like Peptide-1 (GLP-1) agonists are an increasingly used class of medications in the treatment of Type 2 diabetes and obesity. To date, there are only conflicting case reports detailing the clinical effects seen in toxicity to GLP-1 agonists.
Methods: This retrospective, descriptive study was designed to identify and characterize clinical effects of GLP-1 receptor agonist exposures reported to US Poison Centers. Utilizing the National Poison Database System (NPDS), data regarding single substance exposures to GLP-1 agonists between 1/1/2005 and 12/31/2016 were gathered.
Results: In total, 1,307 single substance exposures followed to a known outcome were identified. The median age of followed cases was 57 years [9 months-90 years] and 73.0% (N=954) of patients were female. Four unique GLP-1 agonists were reported, with exenatide and liraglutide accounting for the majority of exposures. The majority of cases (N=1,049, 80.3%) of cases were the result of an unintentional therapeutic error with inadvertently taken/given a medication twice (N=466, 44.1%) as the most common scenario. The majority of cases were coded as no effect (N=726, 55.5%) or a minor outcome (N=356, 27%). No deaths were reported. Common reported clinical effects were nausea (N=251, 19.2%), vomiting (N=162, 12.4%), and hypoglycemia (N=149, 11.4%).
Conclusion: Results indicate many clinical effects of GLP-1 agonists overdose reported to US Poison Control Centers are similar to adverse effects occurring with therapeutic use, though rates of hypoglycemia appear to be higher. Further studies are needed to determine referral thresholds for GLP-1 agonists.
