Drowne Taylor
Relationship Between Tumor Mutational Burden and Progression-Free Survival in Non-Small Cell Lung Cancer Patients Treated with Immunotherapy
April 23, 2020 in College of Pharmacy, Virtual Poster Session Spring 2020
Abstract.
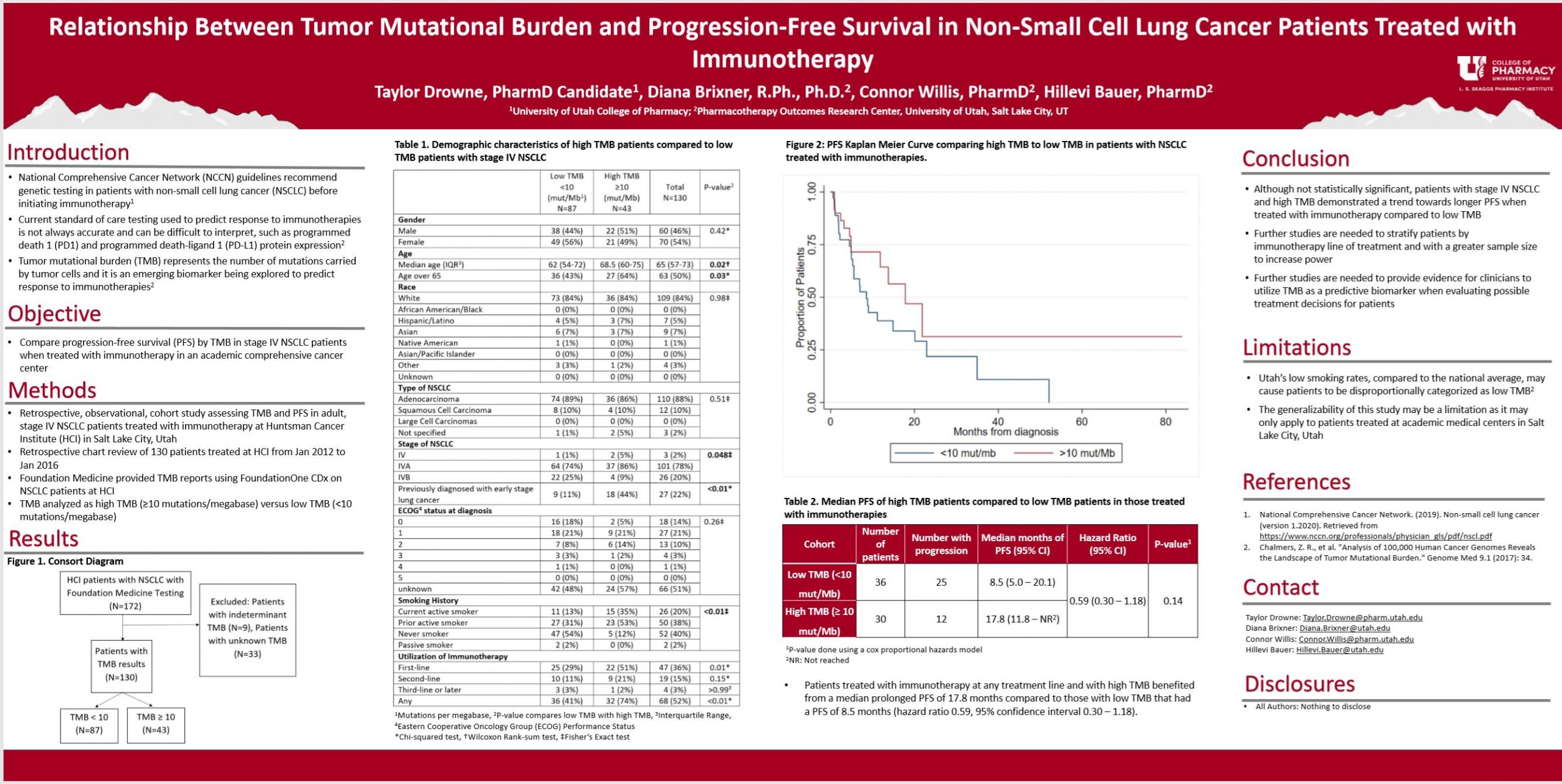
Purpose: Tumor mutational burden (TMB) is an emerging biomarker that represents the number of mutations carried by tumor cells. TMB is being explored as an independent biomarker to predict response to immunotherapies beyond the current standard of care (testing for programmed death-ligand 1 [PD-L1] expression levels). The purpose of this study is to determine if stage IV non-small cell lung cancer (NSCLC) patients with high TMB achieve prolonged progression-free survival (PFS) compared to patients with low TMB when treated with immunotherapy.
Methods: This is a single-site, retrospective, observational, cohort study assessing TMB and PFS in adult, stage IV NSCLC patients treated with immunotherapy at Huntsman Cancer Institute (HCI) in Salt Lake City, Utah. A retrospective chart review of 172 patients treated at HCI was performed. Foundation Medicine provided TMB reports using FoundationOne CDx on NSCLC patients at HCI from 1 January 2012 to 26 January 2016. PFS data was collected from the tumor registry and HCI medical chart review, and results were stratified by TMB. Kaplan-Meier curves were constructed to estimate PFS.
Results: Patients with high TMB treated with immunotherapies experienced a median PFS of 17.8 months compared to patients with low TMB that had a median PFS of 8.5 months. These results validate previous studies that explored a positive association between TMB and PFS with immunotherapies.
Conclusion: Patients with stage IV NSCLC and high TMB benefit from a prolonged PFS compared to patients with low TMB when treated with immunotherapies. Further studies are needed to provide evidence for clinicians to utilize TMB as a biomarker when evaluating possible treatment decisions for patients with stage IV NSCLC.
