Effect of Sacubitril-Valsartan on Loop Diuretic Dose in a Real-World Heart Failure Population
Introduction:
Sacubitril/valsartan is an angiotensin-receptor neprilysin Inhibitor (ARNI) that reduced the risk of death or hospitalization for heart failure vs enalapril in the PARADIGM-HF trial. Sacubitril/valsartan increases natriuresis and diuresis by inhibiting the breakdown of vasoactive peptides. In a post-hoc analysis of the PARADIGM-HF trial, patients on sacubitril-valsartan had overall decreased diuretic use compared to patients on enalapril. This study tests this hypothesis in a real-world population of heart failure patients.
Research Question:
This study compared the absolute change in loop diuretic dose at 6 months between patients started on sacubitril/valsartan and similar patients who started or increased their dose of ACE-I or ARB within the University of Utah Heart Failure and Recovery Clinic between January 1, 2016 and December 31, 2018.
Study Design:
Retrospective cohort study.
Methods:
Patients 18 to 89 years prescribed sacubitril/valsartan or who started/increased their dose of ACEi or ARB, with left ventricular ejection fraction ≤40%, and who are seen in the University of Utah Heart Failure and Recovery Clinic were identified via Epic Hyperspace Reports and reviewed for inclusion. If the sacubitril-valsartan, ACEi, or ARB was discontinued or the patient received a heart transplant or left ventricular assist device during the 6 month period, the patient was excluded. Loop diuretic doses at 0 and 6 months, and other patient characteristics were obtained via chart review. The primary endpoint is absolute change in loop dose. Secondary endpoints are percent of patients needing a loop dose increase and percent needing a decrease. The primary outcome was analyzed using a t-test (if the data are normally distributed or a non-parametric test if not).
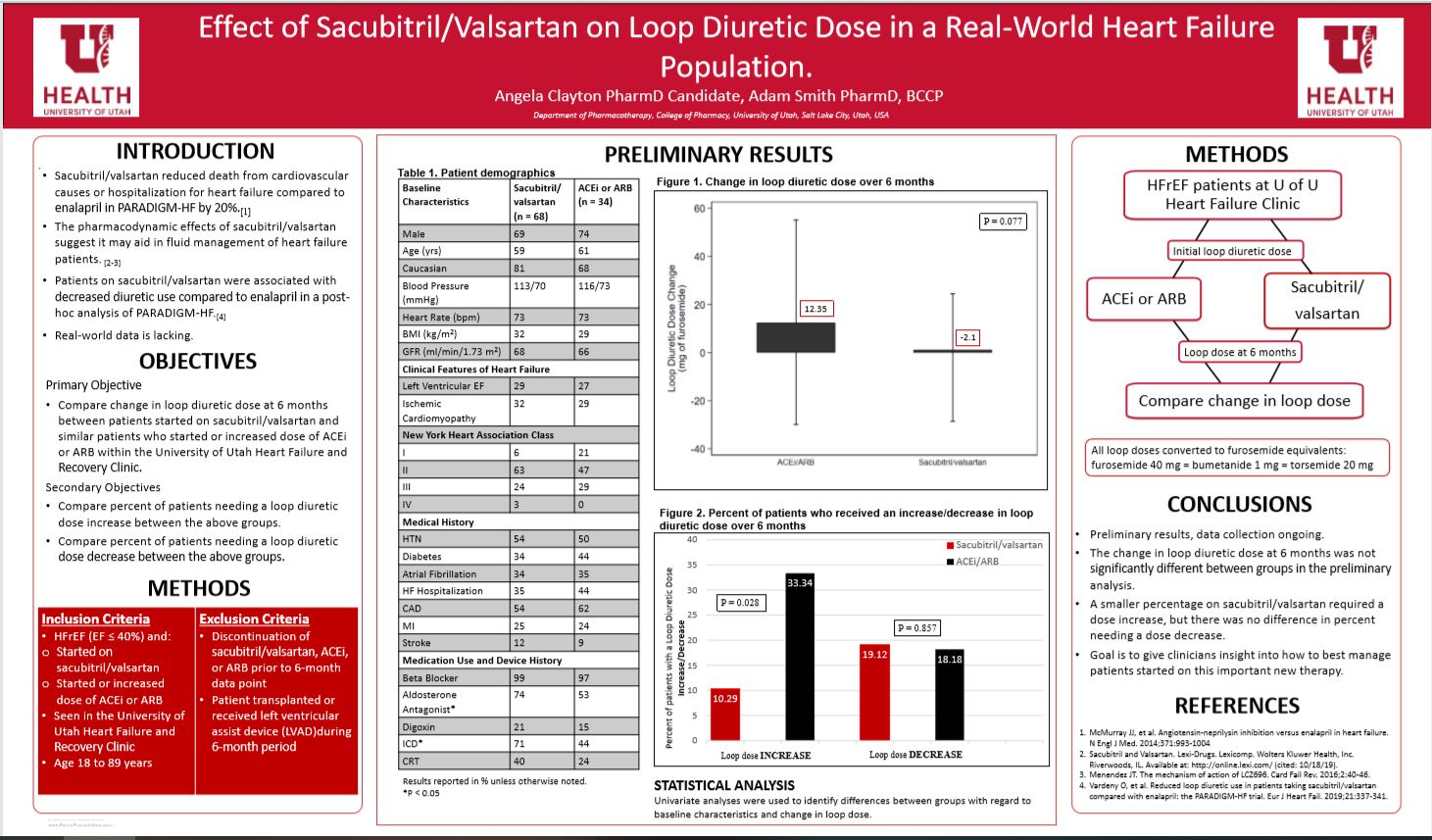
Results:
Preliminary data showed the change in loop diuretic dose for the ACEi/ARB group was 12.35 and for the sacubitril/valsartan group was -2.1 (p=0.077) this does not meet statistical significance between groups. However, only 10.29% of sacubitril/valsartan required a dose increase, compared to 33.34% in the ACEi/ARB group (p=0.028). There was no difference in percent needing a loop diuretic dose decrease.
Conclusion:
Per preliminary results sacubitril/valsartan does not have a statistically significant net reduction in loop diuretic dose compared to the ACEi/ARB group. Sacubitril/valsartan required less of a loop diuretic dose increase compared to the ACEi/ARB group. More information is necessary to determine sacubitril/valsartan’s effect on loop diuretic dose.
Published in College of Pharmacy, Virtual Poster Session Spring 2020
Interesting study. Thank you.
Thank you for the interesting poser. Did you by chance look at length of diuretic therapy prior to starting sac/val vs ACEi/ARB? I was curious to know how diuretic dependent the patients were with dose differences between the two groups. did the patients who start sac/val have a higher or lower mg furosemide equiv starting out? also thought it was interesting the ACE/ARB group had nearly 47% of patients not on an aldosterone antagonist. interested to know if there was a difference in that group alone without full RAAS blocking therapies. Thank you
Those are good questions however, we didn’t note how long patients were on diuretics before starting sac/val or ACEi/ARB some weren’t on a diuretic for the initial dose logged. We also didn’t note which group had a higher/lower furosemide equiv but that is something we may look into when we finish collecting our data. The aldosterone antagonist use was lower in the ACEi/ARB group we believe that is because patients starting sac/val at the cardiovascular clinic they try to optimize their HFrEF therapy as tolerated. These are good questions that we may look into once we finish collecting the data, it would be interesting to answer some of these questions.
Angela, Nicely presented. Your preliminary findings look promising in relation to your overall hypothesis. Do you think the differences in other medications/devices may contribute to any potential differences in you primary and secondary outcomes?
Thanks for reviewing my poster Dr. Keefe, we do think that the use of aldosterone antagonist being higher in the sac/val group could potentially cause differences in our primary and secondary outcomes. Although, we did consider this when developing our study, we didn’t find a way to avoid it other then making the study as random as we could.