Characterization of adverse effects of GLP-1 receptor agonist exposures reported to US Poison Centers
Abstract:
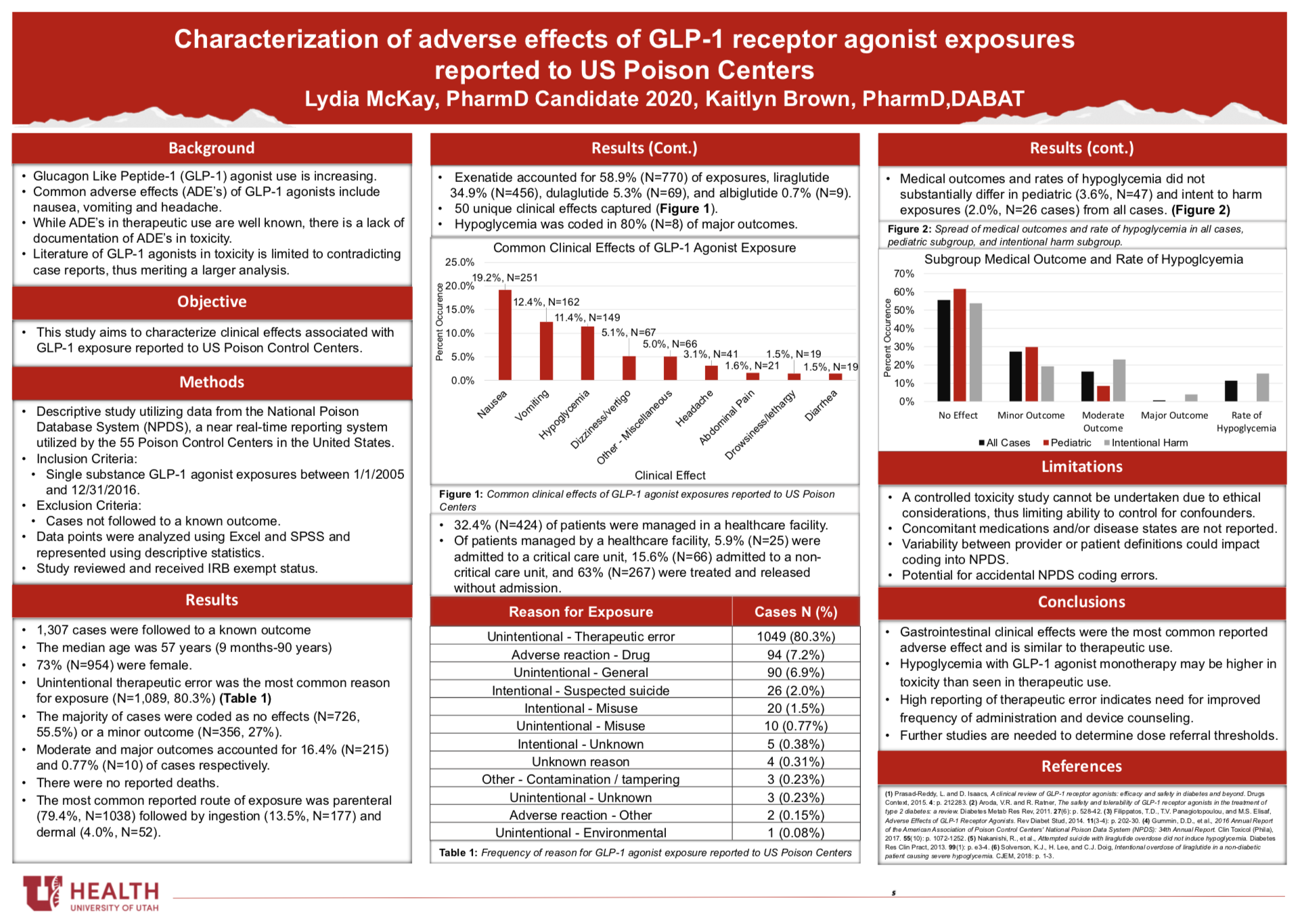
Background: Glucagon Like Peptide-1 (GLP-1) agonists are an increasingly used class of medications in the treatment of Type 2 diabetes and obesity. To date, there are only conflicting case reports detailing the clinical effects seen in toxicity to GLP-1 agonists.
Methods: This retrospective, descriptive study was designed to identify and characterize clinical effects of GLP-1 receptor agonist exposures reported to US Poison Centers. Utilizing the National Poison Database System (NPDS), data regarding single substance exposures to GLP-1 agonists between 1/1/2005 and 12/31/2016 were gathered.
Results: In total, 1,307 single substance exposures followed to a known outcome were identified. The median age of followed cases was 57 years [9 months-90 years] and 73.0% (N=954) of patients were female. Four unique GLP-1 agonists were reported, with exenatide and liraglutide accounting for the majority of exposures. The majority of cases (N=1,049, 80.3%) of cases were the result of an unintentional therapeutic error with inadvertently taken/given a medication twice (N=466, 44.1%) as the most common scenario. The majority of cases were coded as no effect (N=726, 55.5%) or a minor outcome (N=356, 27%). No deaths were reported. Common reported clinical effects were nausea (N=251, 19.2%), vomiting (N=162, 12.4%), and hypoglycemia (N=149, 11.4%).
Conclusion: Results indicate many clinical effects of GLP-1 agonists overdose reported to US Poison Control Centers are similar to adverse effects occurring with therapeutic use, though rates of hypoglycemia appear to be higher. Further studies are needed to determine referral thresholds for GLP-1 agonists.
Published in College of Pharmacy, Virtual Poster Session Spring 2020
Lydia (and Kait!). Nicely done, both in the science and in the presentation on the poster! This is quite interesting, and it seems that your conclusion about needing clearer instructions for patients to decrease therapeutic dosing errors is spot-on. I’m curious about the nature of the issues that led some patients to the ICU or needing more care. Were those largely intentional over-doses/suspected suicides, or were there patients who made a therapeutic error and had a really adverse response? Other?
Hi Dr. Keefe,
Great questions! For patients that were admitted to either a critical care or non-critical care unit (total N=91), 58.2% of were the result of an unintentional therapeutic error. The other top reasons for patients needing admission were adverse drug reaction (14%, N=13) and suspected suicide (13%, N=12). Based off those numbers, unintentional therapeutic error can potentially require hospital admission. That number may be biased in that there were substantinally more cases of therapeutic error with these agents than those with intent to harm.
What I think is really interesting, is that of those 91 patients admitted to the hospital, 30.8% (N=28) were recorded as having no effect (further broken down into 26.4% minor effect, 38.5% moderate effect, and 4.4% major effect). In talking to Kait, patients can be recommended for admission by PCC staff in the setting of an overdose if adverse effects are anticipated, even if the patient is not currently experiencing them. For example, if there is high concern for hypoglycemia, patients may be admitted to an ICU for hourly glucose checks despite not having a low blood sugar at the time of presentation. Unfortunately, given the nature of the data, we don’t know if there were additional confounding factors (e.g. comorbidities) that also played into the decision to admit the patient.
Let me know if you have any other questions!
Fascinating study! As you had alluded to in your conclusions, I think this gives us a better picture of the potential toxicities that can occur with GLP-1 agonists without having to do a toxicity study.
Thanks Angie!
Just to clarify, were there 47 cases of pediatric hypoglycemia? The figure makes it look like there were none.
If there were any pediatric cases, were <18 y/o counted as pediatric or <6 y/o? If there were any young children who became hypoglycemic do you have details on the cases at all?
Great project and hope it makes it to NACCT!
Hi Dr. Moss,
Thank you! There were a total of 47 pediatric cases (counted as less than 18 years) reported to poison control centers. There were no reports of hypoglycemia in any of the pediatric patients. The majority of pediatric cases had no effect (61.7%) followed by minor at 29.8% and moderate at 8.5%. There were no major effects within the pediatric group.
I wish I could say that, given these outcomes, there is a lower risk with exposure to these agents in pediatric patients, but I think the sample size is too small in order to make any clear conclusions. I did briefly look at clinical effects within that subgroup, but didn’t pull numbers. The most reported were nausea and vomiting (aside from puncture wound from the needle).
Please let me know if you have any other questions!
Interesting study! I’m surprised by the number of cases. Most of the GLP-1 pens are pretty easy to use. This may highlight the need for better counseling/instruction for use.
Thank you!
Great job, Lydia!
Thank you!
This is interesting and should inform our work in am care as these agents become more common.
Thank you! I’m curious to see how these results might change now with oral semaglutide as well.
Great job Lydia! It is interesting that there are so many cases, but provides an opportunity for us as pharmacists to make an impact on proper administration and reduction of adverse effects. We as pharmacists can use our knowledge and resources to ensure that the patients understand proper administration and have the tools to assist them in remembering when they take the medication.
Thanks Robbie! I didn’t have room for it on the poster, but the most common scenario for exposure was accidentally taking a medication twice followed closely by taking doses too closely together. I think patients are so used to have daily dosing that these weekly dosed agents can really throw people off. I agree in that pharmacists have a large role in counseling patients with these medications.
Lydia- great study! I found it especially interesting that the rate of hypoglycemia was almost as high as the rate of vomiting in Figure 1 and that most reports were with Victoza (versus a once weekly GLP1 which I would anticipate could easily be confused and administered daily by accident). I’m not sure if you collected any dosing information, but I was curious about what doses were associated with the incidences of hypoglycemia?
Hi Dr. Raber,
Thank you! Exenatide actually had the highest number of reported exposures. However, that being said, it came out much earlier than liraglutide did and I didn’t examine rates of reports over the time frame. I did look at dosing information (below). The dosing ranges were extremely broad. Unfortunately, with this type of data, doses are not reliable. PCC staff sometimes have to estimate doses as best as possible and patients are not always truthful. We don’t know what other factors may have influenced this effect as well (concomitant medications, disease states, etc). That being said, each of the agents that had cases of hypoglycemia experienced that effect at normal, therapeutic doses.
The dose range of cases involving hypoglycemia:
Exenatide: 5 mcg to 30,000 mcg
Liraglutide: 1.2 mg to 15 mg
Dulaglutide: 0.75 mg to 3 mg
There were no cases of hypoglycemia reported with albiglutide.
Please let me know if you have any further questions!
Nice presentation and great poster. Sure excited you’ll be finishing soon! Congratulations!
Thank you!
Lydia, really interesting poster, good job. 1300+ exposures, and no deaths. i am really surprised by that finding.